Abstract
Background. Persistence of measurable residual disease (MRD) is a poor prognostic factor and predicts relapse in acute myeloid leukemia (AML). In a phase I study, the allogeneic leukemia-derived dendritic cell vaccine, DCP-001, has shown safety and humoral and cellular immune responses (A.A. van de Loosdrecht, et al. Cancer Immunol. Immunother. 2018;67:1505). In the current phase II study, (Clintrials.gov: NCT03697707) we report on progress and evaluation of MRD conversion, as primary endpoint, relapse free and overall survival.
Methods. AML-patients, ineligible at screening for HSCT, who are in first complete remission (CR1) but with MRD receive 4 biweekly doses of 25e6 or 50e6 cells per vaccination (cells/vc) followed by 2 doses of 10e6 cells/vc as boosts at week 14 and 18. MRD is assessed at 4 timepoints (baseline, week 14, 20 and 32) by flow cytometry and/or molecular analyses. Primary endpoints are the effect of vaccination on MRD status and safety and tolerability of the two vaccination schedules. Secondary endpoints include relapse free, overall survival and immunological response evaluation.
Results. As of 28 th July 2021, 19 patients have been enrolled, of which 10 patients have been given at least 4 vaccinations with 25e6 cells/vc and 9 with 50e6 cells/vc. No serious adverse events (AE) or severe AE (grade 3 or higher) related to DCP-001 have been reported. AE's related to DCP-001 are mainly injection site reactions, occurring within 48 hours after intradermal administration.
In the 25e6 cells/vc cohort all patients have completed the treatment phase up to week 32, allowing full assessment of MRD response. Three patients have converted to MRD negative, 4 patients relapsed (followed by HSCT for 1 patient), 3 patients remained MRD positive, of which 2 patients underwent HSCT immediately after completion of the full dosing schedule. In this cohort 2 patients eventually died during follow up. Treatment is still ongoing in the 50e6 dose cohort, with thus far one additional MRD conversion reported and two relapses, 3 patients with stable MRD levels, and 3 have no MRD data available yet.
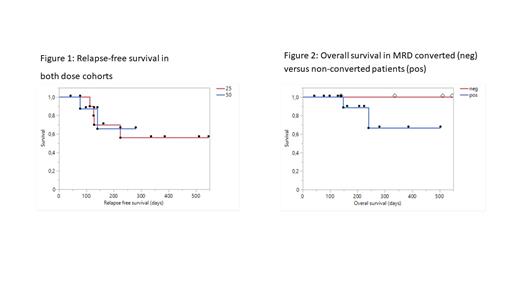
Median follow-up of patients in the 25e6 cells/vc cohort was 288 days (range 148 - 546). Median RFS and OS have not yet been reached, but estimated RFS and OS at 12 months is calculated at 57% and 79%, respectively (Figure 1).
MRD converted patients after DCP-001 vaccination, showed improved survival, compared to patients without MRD conversion (Figure 2). All 4 patients who converted to MRD negative are still in CR and alive (FU median 423.5 days (range 140 - 546).
Immunological monitoring of patients is currently being performed. As previously reported (abstract #168, ASH2020), 3 of 4 evaluable patients show at least 1 or more responses against tumor-associated antigens known to be present in DCP-001 in IFNy ELISPOT assay.
Conclusion/discussion. Four patients have shown MRD conversion (3 in the 25e6 dose cohort, and 1 in the 50e6 dose cohort). Six patients remained in complete remission with stable or declining levels of MRD, 6 patients relapsed and for 3 patients no MRD data is available yet. Median RFS and OS have not yet been reached. MRD conversion showed improved relapse free and overall survival. Treatment with DCP-001 is very well tolerated, with limited side effects mainly related to intradermal administration. Immunological analyses for specific tumor-associated antigen responses and general immune profiling are currently being performed. Preliminary data from this study shows that the relapse vaccine DCP-001 is a promising treatment for patients with AML in complete remission but with residual disease aiming to deepen responses and prolong survival. Its excellent safety profile allows for future combination therapy.
Van de Loosdrecht: Novartis: Consultancy; Alexion: Consultancy; Roche: Consultancy; Amgen: Consultancy; Celgene: Consultancy, Research Funding. Cloos: Takeda: Research Funding; Novartis: Consultancy, Other, Research Funding; Navigate: Patents & Royalties; Merus: Other, Research Funding; Janssen: Research Funding; Helsinn: Other; Genentech: Research Funding; DC-One: Other, Research Funding; Astellas: Speakers Bureau. Platzbecker: AbbVie: Honoraria; Janssen: Honoraria; Novartis: Honoraria; Celgene/BMS: Honoraria; Geron: Honoraria; Takeda: Honoraria. Holderried: Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Speakers Bureau; MSD: Speakers Bureau; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Therakos: Other: Travel support; Abbvie: Other: Travel support; Medac: Other: Travel support; Eurocept Pharmaceuticals: Other: Travel support; Janssen: Other: Travel support; Daiichi Sankyo: Other: travel support. Giagounidis: Novartis: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. van Zeeburg: Immunicum AB: Current Employment; Kiadis: Ended employment in the past 24 months. Rovers: Immunicum AB: Current Employment. Gjertsen: KinN Therapeutics: Current holder of stock options in a privately-held company; Alden Cancer Therapy: Current holder of stock options in a privately-held company; Pfizer Inc.: Consultancy; BerGenBio: Consultancy; Novartis: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal